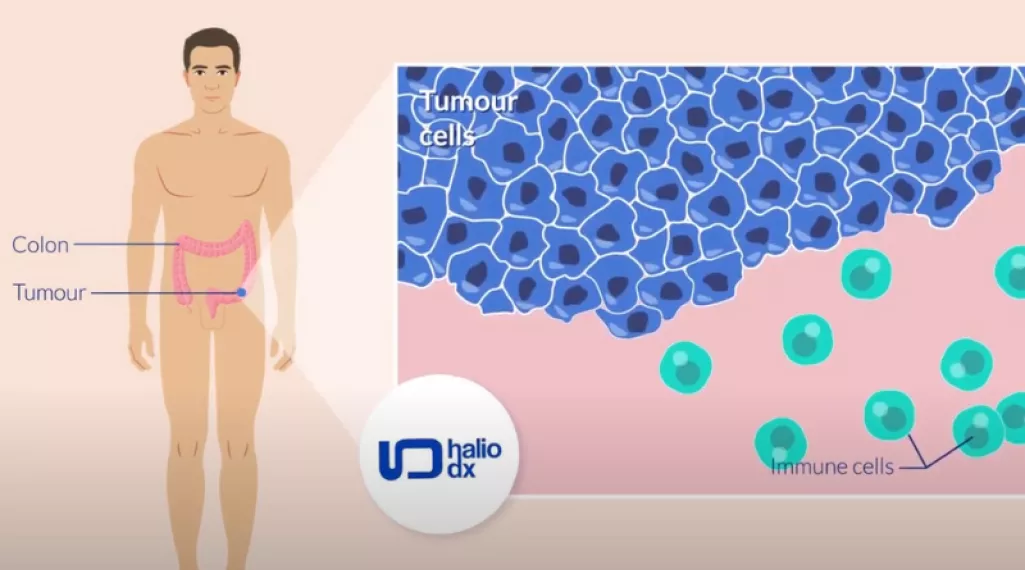
What is Immunoscore?

Immunoscore is the first test available in clinics to measure the response of a patient’s immune system to a tumor. This new technology could refine the prognosis of stage II and some low-risk stage III colon cancer patients and provide doctors with additional information when making decisions about chemotherapy treatment.
Immunoscore measures how well the body’s immune cells, including lymphocytes, surround and enter a tumor. When used with TNM scoring, which is the method doctors commonly use to assign staging to a disease, Immunoscore appears to be a promising way to predict a patient’s risk of recurrence, which can help develop a personalized treatment plan.
The scores provided by Immunoscore, which clinicians may refer to as a biomarker, can be high or low. A high level of immune cell infiltration (Immunoscore-High) indicates that a patient’s immune system is active in fighting a tumor and indicates a lower risk for recurrence. A low Immunoscore (Immunoscore-Low) indicates that a patient’s immune system is not as active in controlling the tumor and suggests a higher risk for recurrence.
Immunoscore appears to be a promising biomarker, and the European Society of Medical Oncology recently added the test to its 2020 guidelines as an option for personalized medicine.
Multiple clinical studies have demonstrated that Immunoscore may predict which patients are likely to experience a recurrence, and it may help identify stage-II patients with “high-risk clinical features” to avoid unnecessary chemotherapy. However, a second clinical trial is ongoing to determine if Immunoscore can reliably predict which patients will respond to chemotherapy.
Immunoscore was pioneered by Jérôme Galon at the Cordeliers Research Center in Paris, with development and commercialization by HalioDx. To learn more about Immunoscore, watch this video on YouTube.
Top resources

Where breakthroughs begin: Project Cure CRC spotlight on Dr. Lisa Mielke
hrough Project Cure CRC, the Alliance is fueling bold, early-stage research with the potential to transform colorectal cancer treatment. Dr. Lisa Mielke’s groundbreaking work explores how the gut’s immune system and nerve signaling influence cancer growth—opening the door to new therapeutic approaches, including repurposed existing drugs. This is what’s possible when promising ideas get the support they need to move forward.

Bringing biomarker testing within reach: CLEAR for CRC to empower patients from day one
Biomarker testing can guide colorectal cancer treatment and improve outcomes. Learn how CLEAR for CRC is helping patients access this critical tool.

John E.: Biomarker testing uncovered a pivotal treatment option
After a grim prognosis, biomarker testing revealed a targeted treatment option for John E. Learn how knowing your biomarkers can change what’s possible.





