Hepatic Artery Infusion Therapy for Colorectal Cancer that has Spread to the Liver
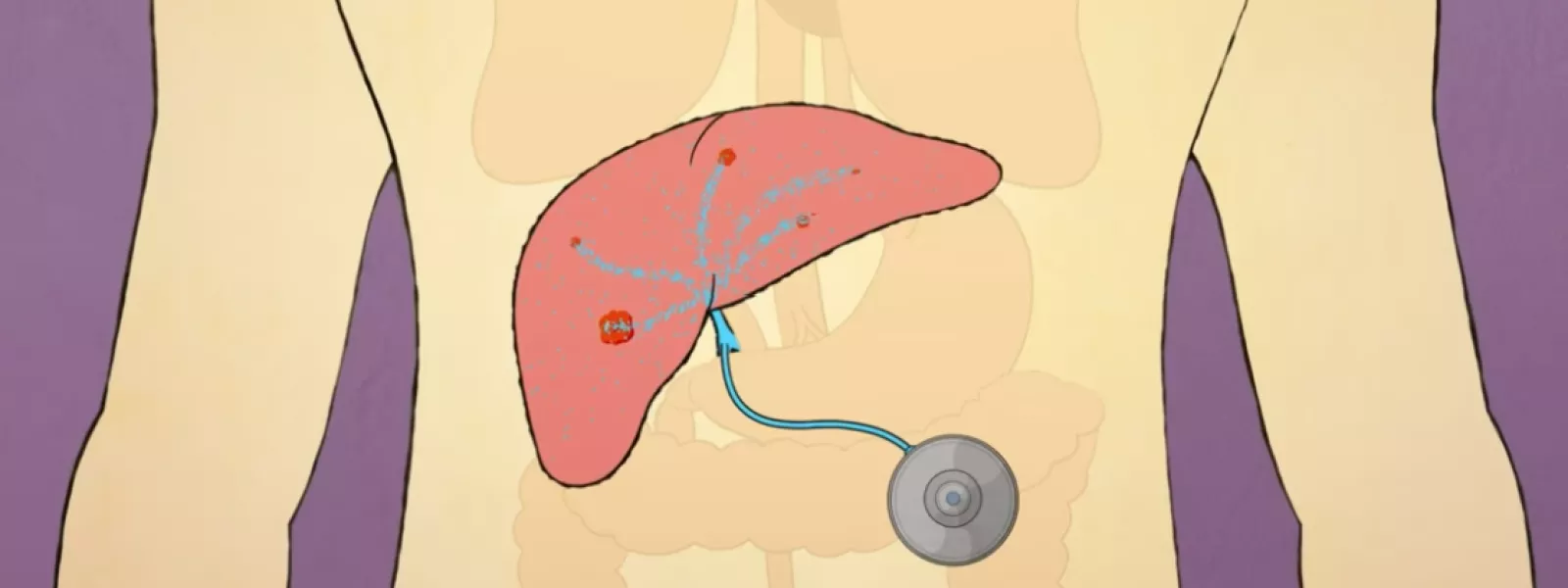
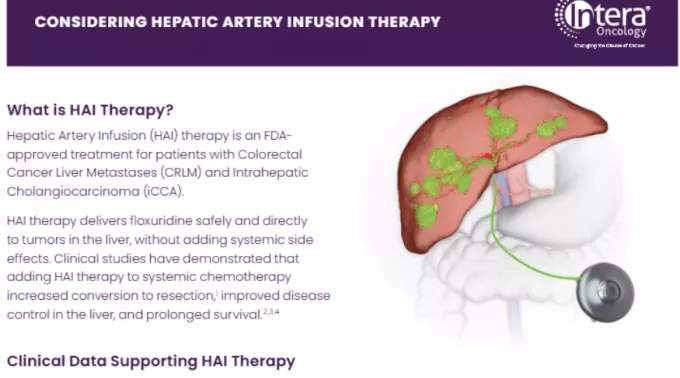
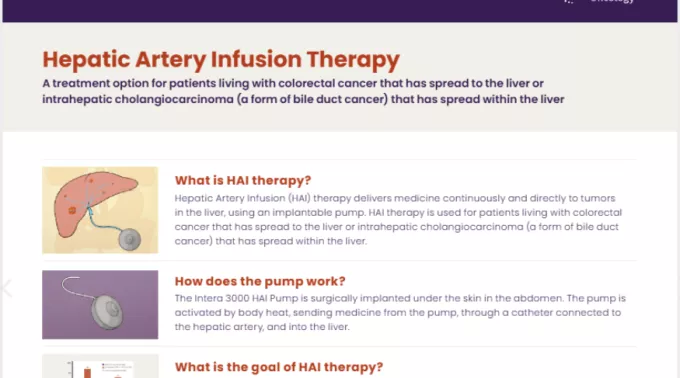
Hepatic Artery Infusion (HAI) therapy is an FDA-approved cancer treatment that delivers medicine continuously and directly to tumors in the liver, using an implantable pump. HAI therapy is used for patients living with colorectal cancer that has spread to the liver.
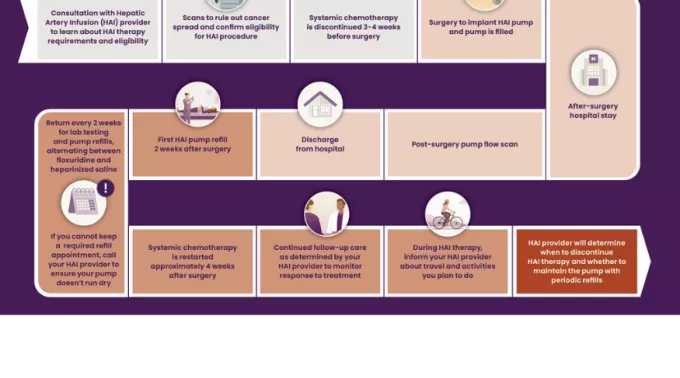
Is HAI therapy right for me?
How does the pump work?
The Intera 3000 HAI Pump is surgically implanted under the skin in the abdomen. The pump is activated by body heat, sending medicine from the pump through a catheter connected to the hepatic artery and into the liver.
What is the goal of HAI therapy?
Clinical studies have shown that HAI therapy may reduce the size of tumors in the liver, improve disease control in the liver, increase chances of liver resection, prevent recurrence, and improve chances of survival.1,2,3,4
How does HAI compare to systemic chemotherapy?
Because the HAI pump connects directly to the liver, the treatment does not need to go through the rest of the body to reach the liver. Unlike systemic chemotherapy, HAI therapy limits potential side effects throughout the body.
Hepatic Artery Infusion: Learn how it works
Resources
Patient-Doctor Conversation Guide
You can provide this overview of HAI therapy to your primary medical oncologist.

What to Ask an HAI Provider
You can use these questions as a guide for your first appointment with an HAI provider.
See Additional Patient Resources

Where can I find an HAI provider?
There are numerous HAI centers across the United States, with more locations opening around the country.
- To learn about an HAI provider near you, fill out this simple form.
- A patient navigator at Intera Oncology, the pump’s manufacturer, will contact you to provide direct information about HAI therapy and help you find an HAI provider.
Patient Testimonials
Read stories of survivors living with the HAI pump.

Hope Brooks: HAI Liver Pump for mCRC Offers Second Chances
Hope Brooks is the vision of health. She exercises daily, teaches core power yoga, and participates in other healthy habits. Weeks after her 50th birthday, a chain of events led to a devastating stage 4 colorectal cancer diagnosis, and Hope researched her condition to look for options. Read more about Hope's story.

Don Shippey: From Stage IV to Six Years Cancer-Free After Receiving HAI Pump
Don Shippey was 55 years old in 2016 when he decided he’d been putting off his colonoscopy long enough. After a stage 4 colorectal cancer diagnosis, Don was determined to search for alternative answers.

Thomas: A New Trajectory with HAI Therapy
Diagnosed with stage 4 colorectal cancer at age 36, Thomas was given months to live. After receiving a second opinion and an HAI pump, his trajectory changed.

Marsha: Being the CEO of Her Own Treatment
After Marsha learned her colorectal cancer had spread to her liver, she found out about HAI therapy and sought a second opinion to decide on next steps.
1. Kemeny N et al. N Engl J Med. 1999;341(27):2039-48.
2. Dhir M et al. Ann Surg Oncol. 2017;24(1):150-158.
3. Groot Koerkamp B et al. J Clin Oncol. 2017;35(17):1938-1944.
4. Pak L et al. J Surg Oncol. 2018;117(4):634-643.
The information provided here is for general informational purposes only and should not be considered as medical advice. Please consult with an HAI provider to determine your specific treatment plan and talk about the potential risks and benefits of the device. Individual results may vary.
The Intera 3000 Hepatic Artery Infusion Pump is indicated for the continuous arterial administration of JND Therapeutics Floxuridine for Injection, USP, heparinized saline, and glycerin. The approved labeling for JND Therapeutics Floxuridine for Injection, USP stipulates the indications, contraindications, and warnings for use of the drug in the pump. The Intera 3000 Hepatic Artery Infusion Pump is contraindicated for use in patients with extensive extrahepatic disease or limited hepatic function. Possible adverse events of the pump are those potential risks associated with any implanted drug delivery device and include: catheter thrombosis, bolus path occlusion, vessel thrombosis, pump dislodgement, seroma, or recurrent hematoma, infection, extravasation, catheter shear, dislodgement or leakage, migration, arterial pseudoaneurysm, arterial dissection, and extrahepatic perfusion. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. Please review the full safety information at https://www.interaoncology.com/patients-caregivers/hai-therapy/safety-information.
This content was developed with support from
Learn more about liver directed therapy
CRCtalks: Liver directed therapies for mCRCTop resources

Where breakthroughs begin: Project Cure CRC spotlight on Dr. Lisa Mielke
hrough Project Cure CRC, the Alliance is fueling bold, early-stage research with the potential to transform colorectal cancer treatment. Dr. Lisa Mielke’s groundbreaking work explores how the gut’s immune system and nerve signaling influence cancer growth—opening the door to new therapeutic approaches, including repurposed existing drugs. This is what’s possible when promising ideas get the support they need to move forward.

John E.: Biomarker testing uncovered a pivotal treatment option
After a grim prognosis, biomarker testing revealed a targeted treatment option for John E. Learn how knowing your biomarkers can change what’s possible.

"Clinical trials gave us time": A daughter’s tribute to her mother’s courage
When Kate Shin’s mother faced rectal cancer, clinical trials gave them precious time together. Now, Kate shares her story to encourage access to screening and innovative care, including clinical trials.






